
Chemical Bonding
Ionic Bonding
Most metal atoms have three or fewer outer electrons. A noble gas configuration is reached if these are lost to form positively charged ions (cations).
Most non-metal atoms have more than three outer shell electrons. To become stable, they must become negatively charged ions (anions).
There are limits to how many electrons an atom can pick up. If one electron is gained, the atom becomes an anion with a negative charge. This will repel any more electrons wanting to join the energy level, and so atoms gaining two or three electrons are rare.
It is also hard to remove three or more electrons from an atom, as the ionisation energy increases after each electron is removed.
When metals bond with non-metals, electrons are transferred from the metal atoms to the non-metal atoms. The metal atoms become cations with a positive charge, and the non metals become anions with a negative charge. Opposites attract, and the atoms are held together by an electrostatic attraction.
Electron dot cross diagrams are used to represent the way the atoms bond together.
Example:
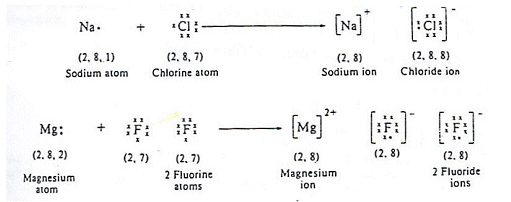 Each sodium atom loses one electron and each chlorine atom gains an electron. The formula for sodium chloride is NaCl, however this doesn't mean that sodium and chlorine are only found in pairs; they are found in lattices.
Each sodium atom loses one electron and each chlorine atom gains an electron. The formula for sodium chloride is NaCl, however this doesn't mean that sodium and chlorine are only found in pairs; they are found in lattices.
The electrostatic force is spread evenly around each sodium cation and chlorine anion. A sodium cation will therefore be able to attract chlorine anions in all directions, and vice-versa.
The chloride cation can attract six sodium anions, therefore the structure formed is a lattice.
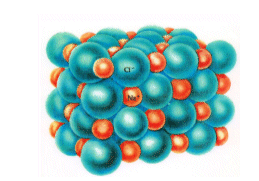 Any sodium ion within the structure will be surrounded by 6 chloride ions. It will be repelled by the other sodium ions, and so the layers will form with alternating sodium and chloride ions.
Any sodium ion within the structure will be surrounded by 6 chloride ions. It will be repelled by the other sodium ions, and so the layers will form with alternating sodium and chloride ions.
Covalent bonding
Non-metallic elements bond with each other by “sharing” electrons. This is called covalent bonding. Shared electrons count as the outer electron for both elements.
In this case the Hydrogen is found in pairs, as there are no charges involved in holding the atoms together, so there is no interaction with the other hydrogen atoms.
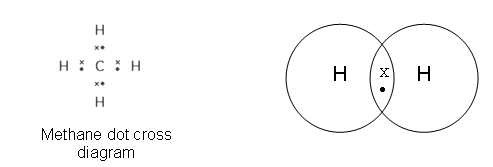
Electron pairs which form covalent bonds are called bonding pairs.
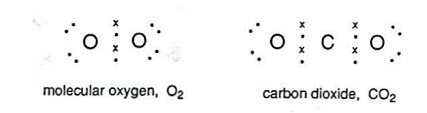 Electrons not involved are called lone pairs.
Electrons not involved are called lone pairs.
When one pair of electrons form a covalent bond, it is called a single covalent bond.
When two pairs of electrons form a covalent bond, it is called a double bond, for example when carbon dioxide is formed.
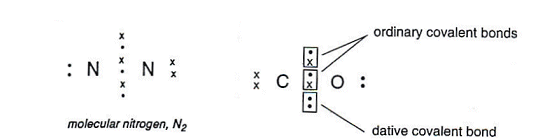 When three pairs of electrons form the bond, it is called a triple covalent bond.
When three pairs of electrons form the bond, it is called a triple covalent bond.
 Two of the bonds in the CO are formed by atoms contributing an electron to the shared pair. In the third bond, both of the electrons come from oxygen, this is called a dative bond.
Two of the bonds in the CO are formed by atoms contributing an electron to the shared pair. In the third bond, both of the electrons come from oxygen, this is called a dative bond.
Polar and non-polar bonds
The atoms in a covalent bond are held together by the nuclei which are exerting a pull on the shared pair of electrons, which are located between the two atoms.
When both atoms that have bonded are the same, the pull on the electrons is the same, so the electron pair is exactly in between the two of the atoms. These are known as non-polar bonds.
Different atoms can attract the electrons unequally, so the electron pair will be closer to one atom than the other.
The atom with the greatest proton pull will have the electrons closer to it. The amount of protons and the distance from the proton will affect the force of attraction. These sort of bonds are called polar bonds.
The “electron pulling” power of an atom is known as its electronegativity. Atoms with strong forces of attraction for the shared pair of electrons are said to have a high electronegativity.
The lower down the group you go; the less the electronegativity is, and the further along the period you go; the higher the electronegatvity the atom has. The electronegativity trend is the same as the first ionisation energy.
 We can use the difference in electronegativity values to predict how polar a covalent bond will be. In a Carbon-Fluorine bond; fluorine has a electronegativity of 4, and carbon has an electronegativity of 2.6, so the pair of electrons will be closer to the fluorine atom.
We can use the difference in electronegativity values to predict how polar a covalent bond will be. In a Carbon-Fluorine bond; fluorine has a electronegativity of 4, and carbon has an electronegativity of 2.6, so the pair of electrons will be closer to the fluorine atom.
This can be written as:
Cd+ -- Fd-
The fluorine has got a slightly negative charge, as the electrons are closer, and carbon has a slightly positive charge, as the electrons are further away.
If the electronegativity is high enough, the electron will be taken fully by the element with the highest electronegativity resulting in ionic bonding.
Metallic Bonding
The strong forces which act between the separate atoms within a metal are known as metallic bonds.
The diagram below explains why metals act as they do:
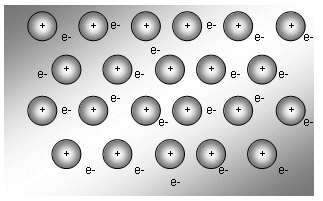 The positive ions are arranged in a regular spaced lattice shape. The outer shell electrons move freely through this lattice. The free electrons are often described as a “cloud” or “sea” of electrons.
The positive ions are arranged in a regular spaced lattice shape. The outer shell electrons move freely through this lattice. The free electrons are often described as a “cloud” or “sea” of electrons.
Each positively charged ion is attracted to the “sea” of negative electrons.
The electrostatic attraction binds the entire structure together as one unit.
In the model above, a particular electron does not belong to one of the positive ions, but is attracted to all of them. These electrons are described as delocalised.
The strength of the metallic bonding depeneds upon the number of electrons. Therefore magnesium (two outer electrons) has stronger metallic bonding than sodium (one outer electron).
This strong electrostatic attraction is why metals have high boiling/melting points, and are dense strong materials.
They are good conductors of electricity due to the delocalised electrons that are mobile.
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home









