
Interaction between Radiation and Matter
Recap from Elements of Life
-
There are lots of different types of electromagnetic radiation. See the electromagnetic spectrum below.
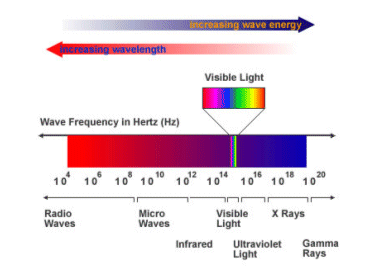
- As the frequency of light increases, its energy also increases. This can be seen in the formula below:
Energy = Planck’s Constant x Frequency of light
- Planck’s constant is equal to 6.63 x 10-34J Hz-1. The higher the frequency of radiation, the higher its energy.
Energy Interacts with Matter
- Electromagnetic radiation can interact with matter, transferring energy to the chemicals involved, making things happen.
- The changes that occur depend upon the chemical involved in the reaction, and the energy involved.
- A molecule has energy associated with four different aspects of its behaviour:
- Translation- the molecule moving around as a whole.
- Rotation- the rotation of the molecule as a whole.
- Vibration- the vibration of the bonds.
- Electronic- electrons moving from one energy level to another.
- These different energy activities require different amounts of energy. The amount of energy required increases down the list.
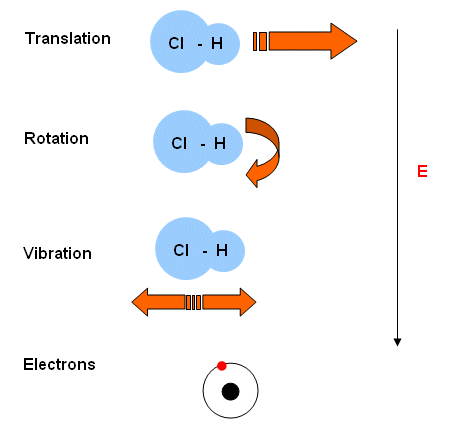
- When the atoms are given energy, their electrons get promoted to a higher energy level. The energy required for the promotion of an electron is specific, it is quantised.
- All other energy is also quantised, i.e. it requires a certain amount of energy to vibrate an HCl molecule, the HCl molecule can have different vibrational energies.
- When its vibrational energy changes, it moves to a new fixed energy level, in a similar way to how electrons are promoted.
- This corresponds to a photon of infrared radiation; we sense radiation energy as heat. The radiation makes the bonds in your skin vibrate more rapidly, and this is why you feel warmer.
- To rotate molecules require less energy than needed to vibrate them. Therefore the rotational energy corresponds to a frequency of light lower than that of infrared radiation, mainly the microwave region.
- The spacing between translation energy levels is even smaller; we treat translational energy as being continuous.
- To make electron changes within the molecule requires a higher energy than is needed to make vibrational changes. Electronic changes involve making electrons shift from a lower energy level to a higher one. Electronic energy changes require energy from the visible and ultraviolet part of the spectrum.
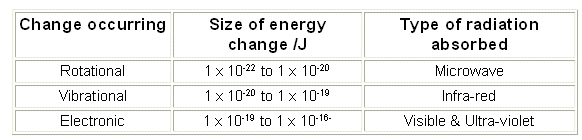
- The particular value of energy required depends upon the chemical involved. Different substances have different structures, and this gives them different energy levels.
- A C-F bond is stronger than a C-Br bond, so more energy is needed to make it vibrate.
Electronic changes due to Ultra-Violet radiation
- Electrons in atoms occupy definite energy levels. Electrons in molecules similarly occupy definite energy levels.
- When the molecule absorbs visible or ultraviolet light one of three things can happen:
- Electrons become excited so they move up from a lower energy level to a higher one.
- If higher energy radiation is used, the two atoms can break apart, they dissociate. Radicals are produced:
Cl2  Cl + Cl
Cl + Cl
- If even higher energy radiation is used, the molecule may lose an electron; it can ionise:
Cl2 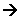 Cl2+ + e-
Cl2+ + e-
Useful books for revision:
Revise AS Chemistry for Salters (Written by experienced examiners and teachers of Salter's chemistry)
Revise AS Chemistry for Salters (OCR) (Salters Advanced Chemistry)
Home




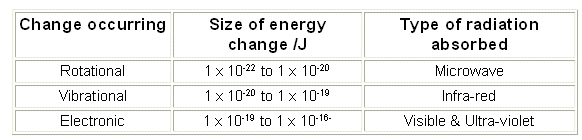
 Cl + Cl
Cl + Cl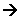 Cl2+ + e-
Cl2+ + e-