
Recrystallisation and Melting point analysis
Recrystallisation
- This is a process that can be used to purify a solid substance. It essentially involves dissolving a substance in an appropriate solvent and then having it come out of the solution in a crystalline form.
The Procedure:
- Dissolve the impure solid into just the right amount of a warm, approproate solvent (until a saturated solution is formed).
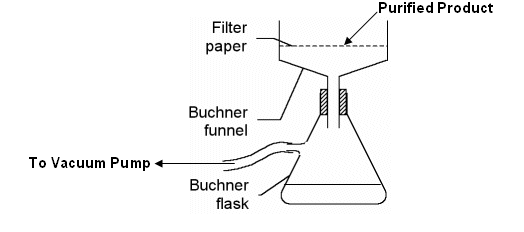
- If the solution is not clear; filter the solution through a buchner funnel to remove any insoluble impurities.
- Cool the solution, so that the product recrystallises leaving the smaller amounts of impurities in the solution.
- Filter the solution to recover the purified product.
- Wash the solid with small amounts of pure solvent to wash away the solution of impurities still on the solid.
-
Allow the solvent to evaporate.
Melting Point Analysis
- Melting point analysis can be used to determine how pure a substance is.
The Procedure
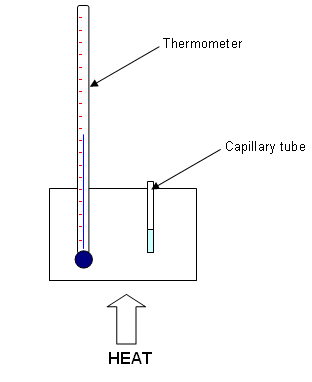
- Fill a melting point capillary tube with a small sample of your purified sample. Tap gently on the bench to ensure that the substance goes to the bottom of the tube. (Fill to around 0.5 cm).
- Insert the capillary tube into the melting point analysis apparatus, and apply heat that will cause the temperature to raise by about 10oC per minute.
- Watch carefully for a sign of melting. Record the temperature range over which the substance has melted.
A small range indicates that the substance is pure. If your substance is impure, you will have a solid that melts over a range of temperatures.
A good target to aim for is a substance that will have a range of a few degrees. If the substance is heated too fast, then the melting point will be higher than expected.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)Salters (OCR) Revise A2 Chemistry