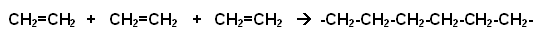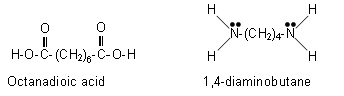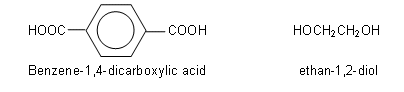Condensation Polymers
-
Polymers are long chains of repeating units known as monomers.
-
Additional Polymers are formed when many identical monomers join together to form a polymer (these polymers can be referred to as A-A polymers), for example:
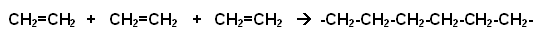
-
Condensation polymers are formed when lots of smaller molecules (monomers) join together to form a larger molecule (polymer), resulting in the loss of small molecules.
-
Condensation polymers are examples of A-B polymers.
Polyamides-
Polyamides are polymers in which the individual monomers are held together by amide linkages.
-
They are made by reacting a diamine with a dicarboxylic acid, or molecules with both an amino group and a carboxylic acid group, for example 1-amino-hexanoic acid.
-
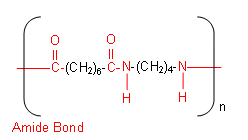
Below is an example of a polyamide:
-
The polyamide is called 4,8 Nylon (there are four carbons on the amine side of the polymer, and eight on the carboxylic acid side).
-
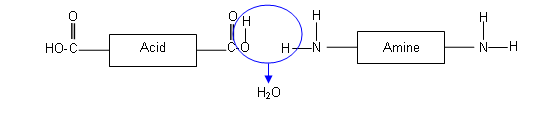
The above polymer is formed when 1,4-diaminobutane reacts with octanadioic acid:
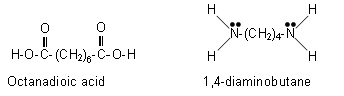
-
These two molecules line up alternatively; forming amide linkages between the adjacent NH2 groups and COOH groups. Each amide bond formed results in the loss of a water molecule.
History of Polyamides- The invention of Nylon
-
Wallace Carothers developed the first ever nylon.
-
He joined the US chemical company Dupont in 1928, where he led a team of investigators working on the production of a polymer that could be used as a fibre.
-
Carothers decided to investigate the polyamides as possible future fibres; however the polyamides formed gummy resins, rather than fibres (due to the presence of water).
-
To get around this problem, Carothers designed a molecular still in which the reaction mixture was heated gently to vaporise the water that was formed.
-
These new polymers were tough, opaque solids; however they melted at low temperatures, meaning that the new fibres could not be ironed. They were also slightly soluble in water.
-
This problem was solved accidentally, when Jullian Hill, a member of Carotherís team, removed a stirring rod from the hot polymer mixture. To his surprise he realised that he had pulled out a long, thin filament. On cooling, the filament could be stretched to many times its usual length.
-
This process was named cold-drawing; it produced fibres that were long, pliable and tough. X-ray diffraction showed that the undrawn fibres were crystalline, but that the crystals had random orientation.
-
Cold drawing causes the polymer chains to become orientated along the length of the fibre. This means that the chains can get closer together and so there are more points where intermolecular forces can act, thus leading to a large increase in the polymerís tensile strength.

-
When the polymer is cold-drawn, a ďneckĒ forms. The polymer chains within this neck line up to form a more crystalline region. The process continues until all of the polymer chains are aligned (except the ends being pulled).
-
Carothers discovered nylon in 1935; he decided that nylon-5,10 had the most potential.
-
However, Elmer Bolton (director of Dupont) argued that nylon-6,6 was the better polymer, due to its high melting point, insolubility in organic substances and its ability to be produced from abundant materials.
-
In 1938, the first product using nylon appeared; a toothbrush with nylon bristles. Nylon stockings were seen for the first time in 1939, along with nylon parachutes.
Polyesters
-
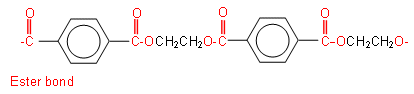
Polyesters are polymers in which the individual monomers are joined together by ester bonds.
-
They are made by reacting a carboxylic acid with two COOH groups, and an alcohol with two ĖOH groups, or molecules with both a hydroxyl and carboxylic acid group, e.g. 4-hydroxy-benzoic acid. The reaction process is called esterfication.
-
Below is an example of a polyester:
-
The polymer chain could continue, as the one unit is repeated many times throughout the section.
-
The above polymer is formed when benzene-1,4-dicarboxylic acid reacts with ethan-1,2-diol:
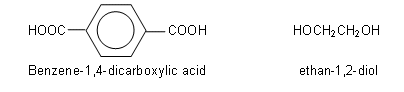
-
These two molecules line up alternatively, forming ester linkages between the hydroxyl and carboxylic acid groups. Each ester linkage results in the loss of a water molecule:
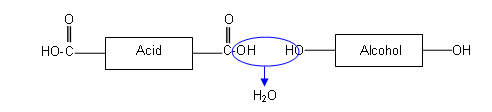
History of polyesters
-
As with the polyamides, the polyesters were first developed by Wallace Carothers.
-
His polyesters were improved by J.R Whinfield and J.T Dickson, who used benzene-1,4-dicarboxylic acid instead of hexanedioic acid. The new polyester they developed was named terylene.
-
Polyesters today are strong, heat resistant and insoluble in water. They are commonly used to produce trousers, skirts, sheets, boat sails and filling materials.
-
The polyester is produced in small granules, which can be melted and squeezed through small holes; producing long fibres known as Dacron or Terylene.
-
Polyesters are now being used to make drink containers; the polyester granules are melted and stretched around a mould.
Kevlar
-
In the early 1960ís, Du Pont were looking for a fibre with the heat resistance of asbestos and the stiffness of glass.
-
The aromatic polyamides seemed to be promising candidates, as the presence of the planar aromatic rings meant that they would form rigid polymer chains, which would require large concentrations of oxygen in order to burn.
-
The first polymeric aromatic amide (aramid) was made from 3-aminobenzoic acid. It could be made into fibres, and whatís more it was fire resistant; however it wasnít very strong (zig-zag nature of chain prevented the molecules aligning themselves properly).
-
Eventually Kevlar was produced. It could be made from readily available starting materials and was cheap to produce.
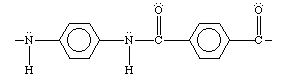
-
Kevlar had to be produced in concentrated sulphuric acid, as it has a high insolubility and needs to be dissolved in a substance in order for the chains to grow.
-
Kevlar is flexible (due to the fibres it forms), light, flame retardant, strong (five times stronger than steel) and has a high insolubility.
-
Kevlarís strength comes from the way the rigid, linear molecules are packed together. The chains align themselves parallel to one another, and are held in place by hydrogen bonds. This leads to sheets of molecules, which stack together regularly around the fibre axis to give a highly ordered structure.
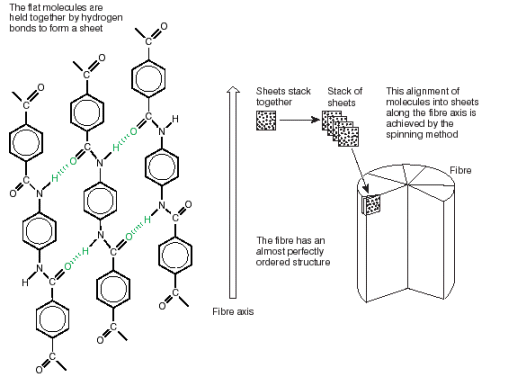
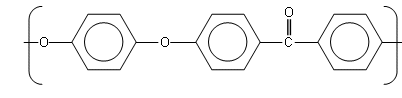
PEEK
-
In the 1960ís, John Rose (a chemist working for ICI Platics) decided to investigate high temperature materials. He wanted to find a material that would have a high melting point and also be resistant to oxidation.
-
Him and his team eventually developed Poly(ether-ether-ketone) (PEEK).
-
PEEK is a polymer which can withstand very high temperatures; it is used to make containers in which you can boil water and as the nose cone of missiles.
Polyhydroxyamine
-
This is a polymer that when strongly heated, turns into a fire-resistant polymer. On burning no smoke is produced, which makes it useful as a plastic for aeroplane interiors.
-
When PHA is heated strongly, it loses water and re-arranges to form PBO. PHA is used as a plastic, rather than PBO, as it is easier to mould.

|