
Designing a Synthetic Route
- It is part of the job of an organic chemist to design a synthesis route for a known compound.
- In order to plan a synthesis route, the chemist must first look at the required compound (the target compound).
- By looking at the functional groups on the target compound, the chemist can work backwards, through a logical sequence of reactions until the suitable starting materials can be found.
- Several steps may be necessary to synthesise the target compound from a suitable starting compound.
- Along the synthesis steps there may be many intermediate compounds that have to be separated and purified from the other compounds.
- In more complex molecules, there may be more than one possible route to the target molecule.
- The preferred route is often the one with the fewest steps; however, other aspects have to be taken into consideration, such as the price of the starting materials.
- In order to design a synthesis route, it is necessary to know the reactions of the functional groups; the organic toolbox is a useful summary of the more common organic reactions.
Example of a Synthetic Route
- Paracetamol is a widely used painkiller that is often used to reduce fever.
- It is more expensive than aspirin; however it is thought to have fewer side-affects and so is a popular choice for many ailments.
- Paracetamol can be synthesised from phenol; using the organic toolkit, it is possible to plan the possible synthesis route.
Planning the Route
-
The structure of paracetamol is as follows:
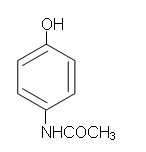
- Looking at the molecule of phenol, the phenolic hydroxyl group which is present on paracetamol is already present and so does not need to be added. To start the synthesis, a nitrogen group needs to be added to the ring. From the aromatic reactions, an NO2 group can be added to the benzene ring in an electrophilic substitution reaction:
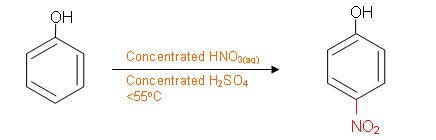
- The NO2 group on the benzene ring needs to be converted to an amide group. This cannot be done in one step; it is first necessary to convert it to a primary amine group:
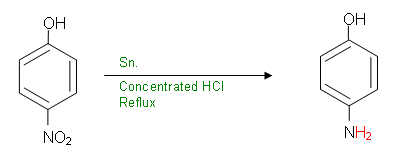
- The ñNH2 group is now prone to an acylation reaction in order to form the secondary amide:
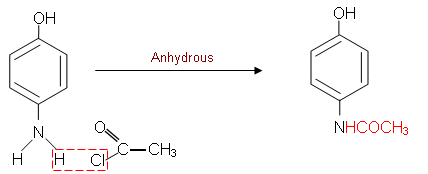
- As is evident in the above synthesis, by looking at the target compound, it is possible to define a synthesis route.
Getting the correct isomer
- The yield of the main product can be lower when there are several isomers of a product molecule formed, and only one is needed.
- For example, electrophilic substitution reactions involving the benzene ring can give a number of isomeric products.
- In the nitration of methyl benzene, there are three possible products:

- The products are all solids, and so the solids must be separated using fractional crystallisation or chromatography.
- Sometimes separating the products can be difficult and time consuming; this all adds to the cost of synthesising the compound.
Useful books for revision
Revise A2 Chemistry for Salters (OCR A Level Chemistry B)
Salters (OCR) Revise A2 Chemistry Home
Home
|





